Compelling body of evidence
Our clinical development program began back in 2013, with hundreds of dogs treated in clinical and case trials to date. Key findings include:
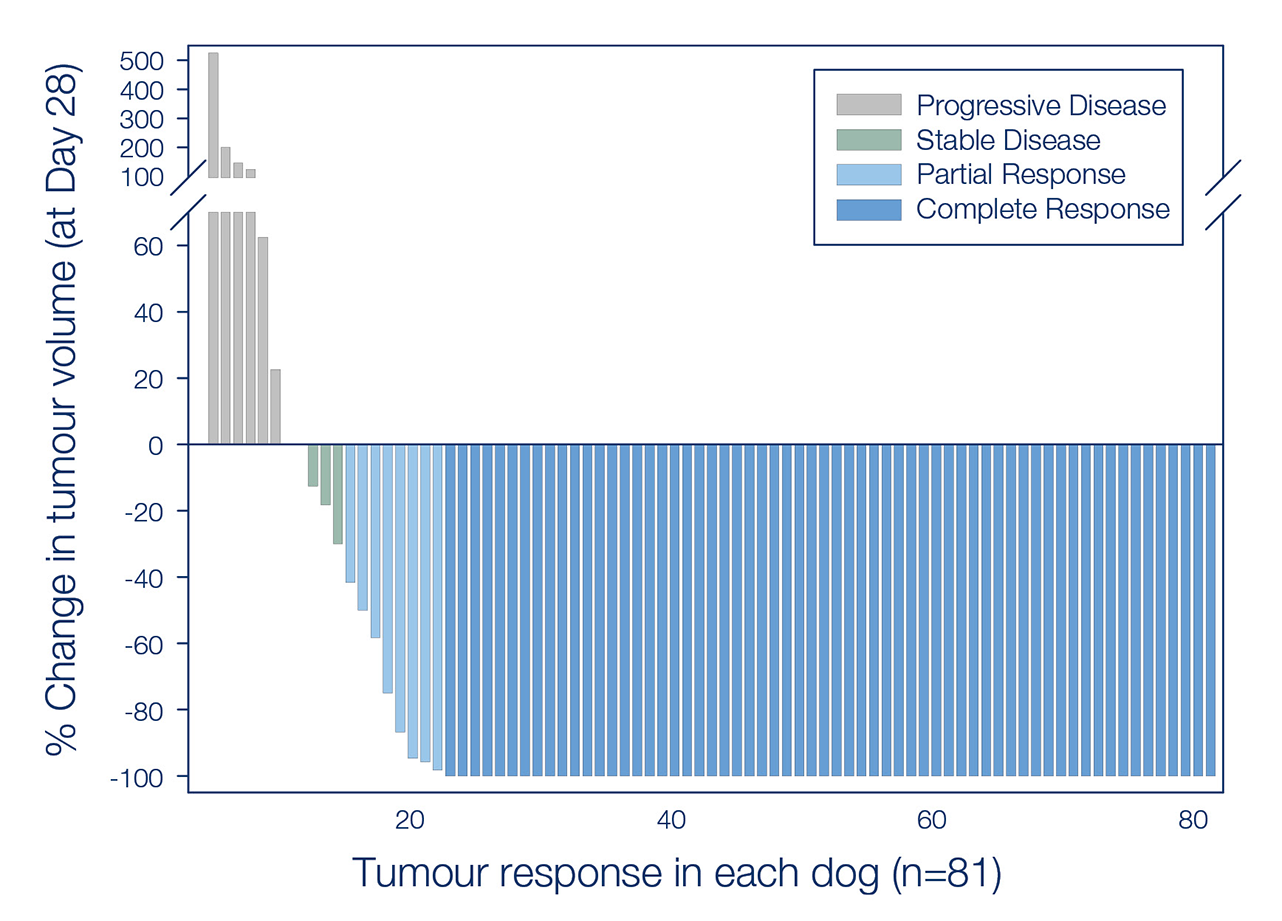
Summary of a pivotal study on 123 dogs to assess the efficacy of a single tiglanol tiglate injection into mast cell tumours.
Robust clinical trial programme
To date, 11 clinical trials have been conducted on solid tumours in dogs including four that were good clinical practice certified.
Randomised, controlled, investigator and owner-blinded, multi-centre clinical study of intratumoural tigilanol tiglate (EBC-46) for the treatment of canine mast cell tumours.
The study of 123 client-owned dogs demonstrated that in those with MCTs, a single intratumoural injection of tigilanol tiglate removed 75% of MCTs at Day 28, significantly higher compared to untreated controls (p<0.001). Further, the trial showed no recurrence in 93% of treated dogs at Day 84. Importantly, the treatment was well tolerated, and animals had a good quality of life during and after treatment.1
Dose Characterisation Study
QBiotics conducted a multicentre, open-label, non-randomised dose de-escalation study in 40 dogs who were assigned to four descending dose cohorts from 1 mg/mL to 0.2mg/mL. Results found 90% of dogs achieved a complete response with 1.0 mL/mL at Day 21. Clinical examinations, serum biochemistry and haematological investigations reinforced the safety profile at all three doses. The peak serum concentration (Cmax) occurred soon after administration (30 minutes) and the mean half-life (T1/2) was short (6.53 hours).3
Publications
- Reddell P et al. Wound formation, wound size, and progression of wound healing after intratumoral treatment of mast cell tumors in dogs with tigilanol tiglate. Journal of veterinary internal medicine, January 2021
- Jones P et al. Recurrence‐free interval 12 months after local treatment of mast cell tumors in dogs using intratumoral injection of tigilanol tiglate. Journal of veterinary internal medicine, December 2020
- De Ridder, T. et al. Use of the intratumoural Anticancer Drug Tigilanol Tiglate in Two Horses. Frontiers in Veterinary Science, 09 September, 2020
- De Ridder TR et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC -46). Journal of Veterinary Internal Medicine, June 2020.
- Hansen N et al. Progressive cutaneous viral pigmented plaques in three Hungarian Vizslas and the response of the lesions to topical tigilanol tiglate gel. Veterinary Medicine and Science; 2018.
- Panizza Benedict J. et al. Phase I dose-escalation study to determine the safety, tolerability, preliminary efficacy and pharmacokinetics of an intratumoral injection of tigilanol tiglate (EBC-46). EBioMedicine, December 2019
- Miller J et al. Dose Characterization of the Investigational Anticancer Drug Tigilanol Tiglate (EBC-46) in the Local Treatment of Canine Mast Cell Tumors.. Frontiers in Veterinary Science, Volume 6, Article 106, April 2019
- Barnett C, et al. Optimising intratumoral treatment of head and neck squamous cell carcinoma models with the diterpene ester Tigilanol tiglate. Investigational New Drugs; April 2018
- Boyle G, et al. Intra-lesional Injection of the Novel PKC Activator EBC-46 Rapidly Ablates Tumors in Mouse Models, PLOS ONE 2017, Vol 9, Issue 10, page number: e108887
- Grant EL et al. Floral and reproductive biology of the medicinally significant rainforest tree, Fontainea picrosperma (Euphorbiaceae) Industrial Crops & Products 108 (2017) 416-422
Presentations
- Lickliter D et al. Phase 1 dose-escalation study of EBC-46 given by intratumoral injection to patients with refractory cutaneous and subcutaneous tumors. Presentation at ASCO Annual Meeting, 2015
- Melo, S et al. Intra-tumoral injection of Tigilanol Tiglate in canine mast cell tumors: Time-assessed thermographic images, computed tomography and clinical response. Presentation at Veterinary Cancer Society Annual Conference, 2019
- Campbell, J et al. Durability of clinical response to intratumoral tigilanol tiglate in canine MCT. Presentation at Veterinary Cancer Society Annual Conference, 2019
- Wiest M. Controlled, Randomized Study of Intratumoral Tigilanol Tiglate (EBC-46) for Treatment of Canine Mast Cell Tumors. Presentation at American College of Veterinary Internal Medicine, June 2019
- Wiest M. Controlled, Randomized Study of Intratumoral Tigilanol Tiglate (EBC-46) for Treatment of Canine Mast Cell Tumors. Presentation at European Society of Veterinary Oncology, May 2019
Tigilanol tiglate was borne from applying the Ecologic™ discovery process
Tigilanol tiglate, is one of our lead small molecules that was developed by applying the QBiotics’ proprietary EcoLogic™ discovery platform. EcoLogic™ is a scientifically based and data driven approach to discovery of bioactive molecular scaffolds with significant therapeutic potential.
EcoLogic™ combines an understanding of the distribution, habitats and ecological interactions of plants, animals and microbes of Australia’s rainforests with a mechanistic understanding of plant defence, signalling and stress-response chemistry. Applying the scientific rigour of EcoLogic™ gives us a well-documented, clearly defined and readily defensible pathway to discovery of new molecules and pharmecueticals, one of which is tigilanol tiglate canine.
References:
- De Ridder TR et al. Journal of Veterinary Internal Medicine 2020;doi:10.1111/jvim.15806
- Melo SR et al. Veterinary Cancer Society Annual Conference 2019
- Miller J et al. Frontiers in Veterinary Science 2019;6(106):1-10.